Can canted coil springs meet FDA regulatory requirements or OEM cleaning validation standards? This question was posed by a client when we recommended using our spring technology in orthopedic surgical instruments.
In this article, we’ll share advanced test data supporting that our canted coil spring meets OEM cleaning validation standards, making it ideal for use in medical devices.
Why Springs Must Meet Medical Device Cleaning Validation Standards
Typically, OEMs validate and verify components like springs during feasibility testing, but feasibility failures can add cost and offset your project timeline.
Advanced test data on components not only helps avoid such feasibility failures, but also speeds development time. To help OEMs stay on track with their medical device development projects, we conducted this advanced testing.
How We Tested Our Springs to Meet OEM Cleaning Validation Standards
For testing, we selected commonly used orthopedic devices that use a single housing with multiple groove configurations consisting of a Bal Seal Canted Coil Spring (BSE 100 series) in each groove.
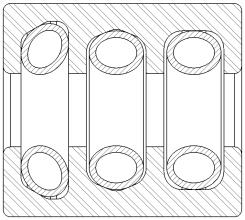
The cleaning validation was performed in accordance with the AAMI TIR30:2011 guidance document, “A compendium of processes, materials, test methods, and acceptance criteria for cleaning reusable medical devices.” The following protocols were used:
- Standard Test Protocol (STP) Number: STP0083 Rev. 10
- Protocol Detail Sheet (PDS) Number: 201305677 Rev.01
Test Soil: Defibrinated blood soil (DBLSO)
Cleaning Method: Manual
Residuals Tested: Hemoglobin and protein
The devices were fully immersed in prepared blood soil. The devices were allowed to remain in contact with the prepared blood soil for a minimum of 15 minutes.
After the 15-minute contact time, the devices were removed from the prepared blood soil and allowed to dry uncovered at room temperature for a minimum of one hour.
Hemoglobin Test
The limit of detection (LOD) for the method was calculated to be 0.16 µg/mL. This value was used in the calculations for test devices with results below the detection limit. The limit of quantitation (LOQ) for the method was calculated to be 0.5 µg/mL. In cases in which the µg/mL value was between the LOD and LOQ, the calculated results were estimated.
The hemoglobin concentrations shown are the average of the three replicates.
| Table I. Hemoglobin test results. | |||
| Article Number | µg/mL | µg/article | µg/cm² |
| 1 | Ë‚0.16 | Ë‚3.2 | Ë‚0.37 |
| 2 | 0.18 | 3.6 | 0.42 |
| 3 | 0.34 | 6.8 | 0.79 |
| Negative article | 1.3 | 25 | 2.9 |
| Positive article | 2.4 | 4,800 | 560 |
Micro BCA™ Protein Test
The LOD for the assay was calculated to be 1.1 µg/mL. This value was used in the calculations for test devices for which the absorbance was below the detection limit. The LOQ for the assay was calculated to be 2.0 µg/mL. In cases in which the µg/mL value was between the LOD and the LOQ, the calculated results were estimated (see Table II).
| Table II. Micro BCA™ protein test results. | |||
| Article Number | µg/mL | µg/article | µg/cm² |
| 1 | Ë‚1.1 | Ë‚2.2 | Ë‚2.6 |
| 2 | Ë‚1.1 | <22 | Ë‚2.6 |
| 3 | 2.3 | 46 | 5.3 |
| Negative article | 1.4 | 28 | 3.2 |
| Positive article | 590 | 12,000 | 1,400 |
Medical Device Cleaning Validation Test Results
Following the cleaning procedure, no soil was seen on the processed test devices under normal lighting conditions during visual inspection. Such testing enables OEMs to validate and verify components like our canted coil springs more quickly and easily.
Because feasibility failures can add cost and time to development, advanced test data on components can help keep development on time and on budget.