
SYGNUS is the world’s first integrated seal and electrical connector system for medical active implantable devices. It’s a plug-and-play solution for the critical lead interface, engineered to help you accelerate development and stay focused on what’s important to your business. The SYGNUS system combines ultra-reliable electrical connector technology with implantable-grade silicone isolation seals to create a serial “stack” that’s both compact and scalable. SYGNUS lets you confidently integrate proven interconnect components AND satisfy demand for smaller, more functional devices—while eliminating the need for dual sourcing or internal component development.
SYGNUS Connector System Construction
The SYGNUS system’s core technology is the electrical connector. Consisting of a housing made from medical-grade MP35N® nickel alloy and a platinum-iridium or MP35N® canted coil spring, the connector offers low insertion force and ultra-low electrical impedance (<100 mΩ). The individual coils of its spring element provide multi-point conductivity, and they automatically compensate for both misalignment and mating surface irregularities.

SYGNUS electrical connector housings are typically cylindrical, but their geometry can be customized to incorporate features that aid in connector retention and simplify assembly processes.
Between the SYGNUS system’s connectors, precision-engineered silicone seals provide superior fluid and dielectric isolation, preventing leakage and current transfer that could cause device malfunction or failure.
SYGNUS is engineered to minimize axial pitch, allowing for greater connector density in less space. Pitch distance, housing diameter, spring diameter, and housing geometry are completely customizable. The SYGNUS system’s connector designs are subjected to comprehensive force testing, and its sealing components are packaged to critical clean standards.
SYGNUS Connector System Sizes
The SYGNUS implantable connector system plays a big role in the consistent, reliable operation of active medical implantables. But it keeps a very low profile, managing more power in less space than any other leading interconnect technology. With our advanced manufacturing processes, we can produce these systems with inside diameters ranging from 0.024 in. (.6 mm) to 0.39 in. (10 mm)+.

SYGNUS Connector System Functions

In devices used to deliver cardiac rhythm management and neuromodulation therapies, the SYGNUS implantable connector system enables the transmission of power from the device battery and electronics to the lead. The highly conductive canted coil spring element in each connector aligns with electrodes on the lead, reliably and consistently managing electrical pulses with minimal heat rise. The system’s silicone seals help prevent fluid ingress, and ensure that channels with different functions don’t interfere with one another. While the SYGNUS system is typically incorporated into the device header, it’s also an ideal solution for inline connections that allow for faster, safer lead replacement.
SYGNUS Connector System Materials
The springs, housings, and seals in our SYGNUS system are available in a range of implantable-grade, biocompatible materials.

SYGNUS Connector System Configurations
We offer the SYGNUS implantable connector system in configurations that meet requirements for current and emerging neuromodulation technologies, as well as industry standards for implantable cardiac devices. These designs can be integrated into your device as a turnkey solution, or they can serve a baseline for accelerated customization.
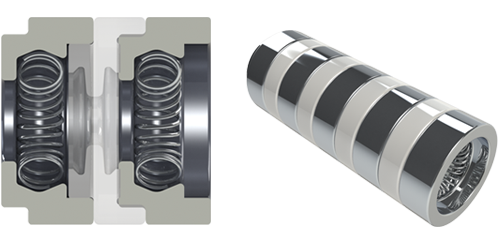 |
|
| 0.9 mm SYGNUS | |
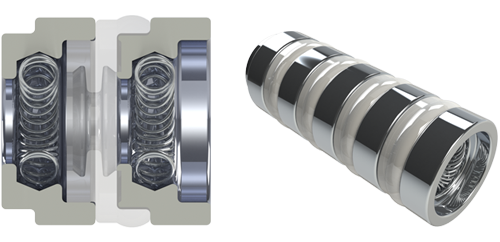 |
|
| 1.3 mm SYGNUS Standard | |
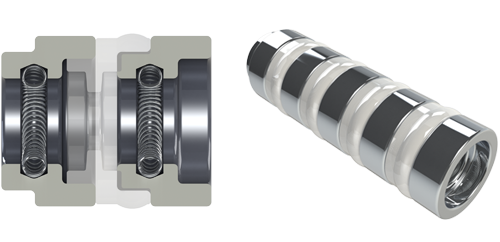 |
|
| 1.3 mm SYGNUS Mini |
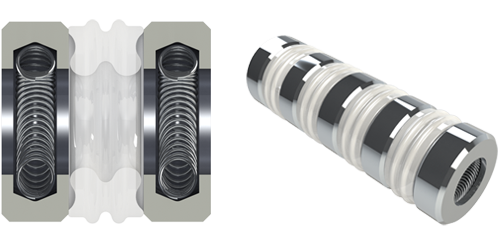 |
|
| 3.2 mm SYGNUS Cardiac |
SYGNUS Connector System Performance
Electrical connector performance can be influenced by size, housing and spring material selection, spring coil density, and many other factors. With our design experience and consultative engineering approach, we can help you make the best choices for your device. Here’s a look at some general SYGNUS® performance characteristics.
Nominal Static Dry Contact Resistance (mΩ)*

Running Force Range (N)

Maximum Breakout Force (N)

Electrical Isolation – Silicone Seal (MΩ)

*Tolerances for this value can range from +/- 15 mΩ to +/- 200 mΩ, depending on a variety of physical and material factors. Contact us, and we’ll help you achieve the optimal electrical performance.
SYGNUS Connector System Spring Force
At the heart of the SYGNUS connector system is our proven canted coil spring technology. Our spring exerts a near-constant force across the working deflection range, and its forces remain consistent. The spring resists compression set, and its individual coils compensate for misalignment, tolerance variations, and mating surface irregularities. The spring’s multiple coils also provide contact redundancy. Spring forces can be engineered to meet minimum/maximum values for lead insertion.








